Viral Vector Manufacturing Market to Reach US$8.0 Bn by 2032 | Persistence Market Research
The viral vector manufacturing market is expanding rapidly, driven by advancements in gene therapies, cell treatments, and vaccine development.
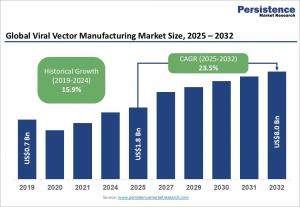
LONDON, UNITED KINGDOM, February 4, 2026 /EINPresswire.com/ -- The global viral vector manufacturing market is on an impressive growth trajectory, expected to be valued at US$1.8 billion in 2025 and reach US$8.0 billion by 2032. This surge represents a compound annual growth rate (CAGR) of 23.5% during the forecast period from 2025 to 2032. The driving forces behind this remarkable expansion include the rapid commercialization of gene therapies for genetic disorders, oncology, and rare diseases, alongside key advancements in bioprocessing technologies, regulatory milestones, and increasing investments in viral vector production capacity.
Download Your Free Sample & Explore Key Insights: https://www.persistencemarketresearch.com/samples/26923
Key Market Dynamics
One of the major factors contributing to this growth is the intensifying commercialization of gene therapies, which are increasingly being used to treat genetic disorders, cancers, and rare diseases. As these therapies progress from research and clinical trials to market-ready treatments, the demand for efficient and scalable viral vector manufacturing solutions is accelerating.
Regulatory frameworks are also evolving to support the advancement of gene therapies. For instance, expedited regulatory pathways, such as the U.S. FDA's Orphan Drug Act and the European Medicines Agency's PRIME designation, allow for faster approval and market access for therapies targeting small patient populations. Additionally, reimbursement models are evolving, with health technology assessment bodies and governments adopting innovative payment structures like installment payments and outcomes-based agreements. This is making high-cost gene therapies more accessible and financially viable.
Virus Type and Application Trends
Adeno-associated virus (AAV) vectors are expected to dominate the viral vector manufacturing market, accounting for approximately 45% of the market share in 2025. AAV’s broad tissue tropism, low immunogenicity, and superior safety profile make it a preferred choice for in vivo gene therapies, such as those used in the treatments of spinal muscular atrophy (SMA), inherited retinal dystrophy, and hemophilia B. Companies are heavily investing in the development of large-scale AAV production capabilities to meet the growing demand.
Lentiviral vectors, however, represent the fastest-growing segment of the market between 2025 and 2032. Lentiviral vectors are gaining popularity due to their ability to transduce both dividing and non-dividing cells, making them essential for cell therapies like CAR-T (Chimeric Antigen Receptor T-cell) and CAR-NK (Natural Killer) therapies. This demand is being fueled by the success of CAR-T therapies like Kymriah and Yescarta, which have demonstrated substantial clinical benefits in treating cancers.
Gene therapy is currently the dominant application within the viral vector manufacturing market, accounting for an estimated 47% of the market share in 2025. This segment is benefiting from a steady increase in the number of approved gene therapies and a robust clinical pipeline addressing a range of genetic disorders, cancers, and rare diseases. The growing clinical approval of these therapies is expected to boost viral vector manufacturing even further.
Viral Vectors in Vaccine Development
Get Custom Insights Designed for Your Business: https://www.persistencemarketresearch.com/request-customization/26923
Regional Market Insights
North America is set to maintain a dominant position in the global viral vector manufacturing market, commanding approximately 54% of the market share in 2025. The U.S. leads the region, backed by its world-class research institutions, extensive venture capital funding, and a regulatory environment that facilitates rapid gene therapy development. The FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation and Priority Review Vouchers have also provided favorable conditions for the advancement of gene therapies.
Europe follows as the second-largest market for viral vector manufacturing, expected to capture 27% of the global market share in 2025. The region’s growth is bolstered by the presence of major pharmaceutical companies and specialized CDMOs in countries like Germany, the U.K., and Switzerland. The European Medicines Agency (EMA) provides a streamlined regulatory process that allows for multi-country clinical development, which has contributed to the region’s attractiveness as a manufacturing hub.
Asia Pacific, however, is the fastest-growing regional market, projected to grow at a CAGR of 22% from 2025 to 2032. The region’s rapid growth is driven by government investments in cell and gene therapy infrastructure, particularly in China, where the government is prioritizing gene therapy production under the "Made in China 2025" initiative. India is also emerging as a significant player in viral vector manufacturing, with companies like Bharat Biotech establishing dedicated facilities for viral vector production to support both domestic and international demand.
Market Challenges and Opportunities
Despite the promising growth outlook, the viral vector manufacturing market faces several challenges. The manufacturing process for viral vectors, especially AAV and lentiviral vectors, is complex and requires adherence to strict Good Manufacturing Practices (cGMP). The complexity of these processes results in higher production costs, which can be a significant barrier for emerging biotech companies and academic institutions. Regulatory compliance requirements, such as batch consistency and potency testing, also add to the financial burden.
However, emerging technologies like AI-powered bioprocess optimization and the adoption of standardized platform technologies are offering transformative opportunities for the industry. AI and machine learning models are being leveraged to optimize manufacturing processes, reducing inefficiencies and improving batch consistency. Standardized platforms that offer pre-validated manufacturing systems are also helping reduce development timelines and production costs, which are essential for scaling production for commercial therapies.
Checkout Now & Download Complete Market Report: https://www.persistencemarketresearch.com/checkout/26923
Market Segmentation
By Virus Type
Adeno-Associated Virus (AAV) Vectors
Lentiviral Vectors
Adenoviral Vectors
Retroviral Vectors
Others
By Application
Gene Therapy
Vaccine Development
Cancer Therapy
Research & Development
Others
By End-user
Biotechnology Companies
Pharmaceutical Companies
Contract Development & Manufacturing Organizations (CDMOs)
Academic & Research Institutes
By Region
North America
Europe
East Asia
South Asia & Oceania
Latin America
Middle East & Africa
Read Related Reports:
CAR T-Cell Therapy Market: The global CAR T-cell therapy market is expected to grow from US$5.8 Bn in 2025 to US$24 Bn by 2032, expanding at a robust CAGR of 25% during 2025–2032.
Medical Tourism Market: The global medical tourism market is expected to reach $2032, growing at a 18.9% CAGR. Cost-effective care & tech-driven treatments fuel demand worldwide.
Persistence Market Research
Persistence Market Research Pvt Ltd
+1 646-878-6329
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.